Which of the Following Compounds Has the Lowest Viscosity
Viscosity is measured using a viscometer. Which of the following substances would you predict to have.

Organic Chemistry Science Educational School Posters Organic Chemistry Chemistry Classroom Teaching Chemistry
CH 3 - CH 2 25 -CH 3 l e.
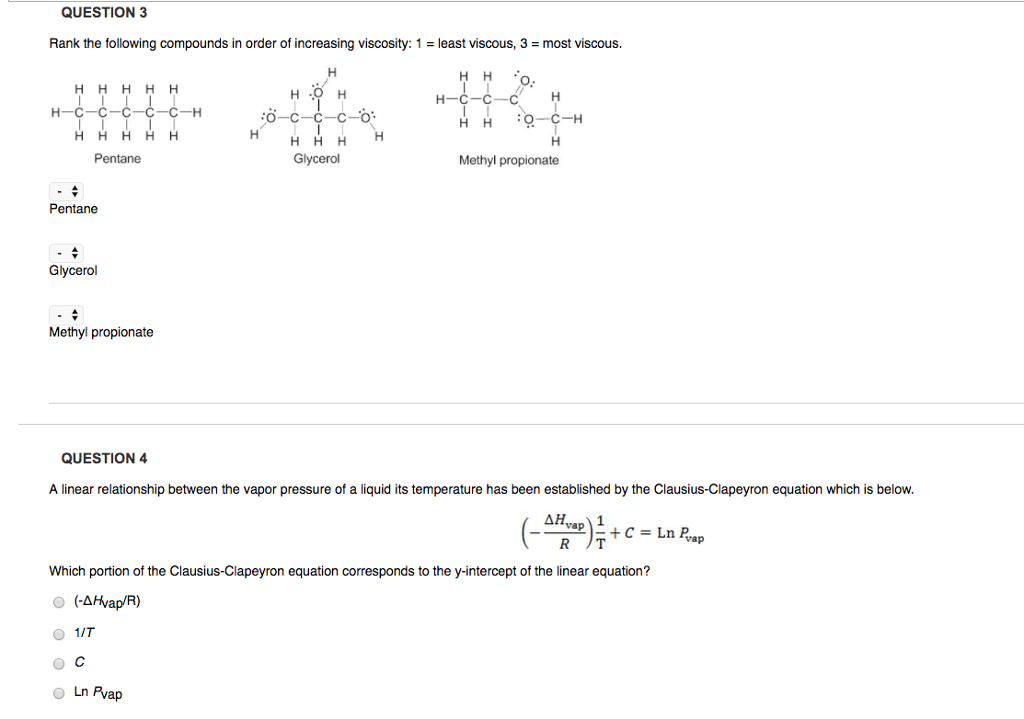
. CHCHCHCH HoCHCHCH and HoCHCHОН. Given below are the temperatures at which two different liquid compounds with the same empirical formula have a vapor pressure of 400 torr. CCl4l N2l H2Ol CH3-CH225-CH3l HCll The normal boiling point of dimethyl ether will be higher than the normal boiling point of ethanol.
N 2 g d. HOCH-CH-CH HOCH. Which of the following compounds has the lowest viscosity.
Steel is considered to be an A. Which of the following pairs correctly ranks the viscosity of the compounds. A CH₃NH₂ CH₃SH CH₃CH₃.
Choose the substance with the lowest viscosity. H2S H2Se AsH3 H2O. Provide an explanation for the following physical properties.
Ons dia O CHCHCHCH HOCHCHOH HOCHCHCH ОНОСНСНОН HOCHCHCH CHCHCH-CH o HoCHCHCH HOCHCHОН CHCHCHCH O HOCH2CH2OH CH3CH2CH2CH3 HOCHCHCH O CHCH-CH-CH. A Water beads up on your windshield but acetone doesnt. N2g OC CH3-CH225-CH3 Od.
Choose the substance with the highest vapor pressure at a given temperature. It corresponds roughly to the intuitive notion of a fluids thickness. Which of the following liquids has the LOWEST viscosity.
A CH₃CH₂OH CH₃OCH₃ CH₃CH₂CH₃. Correctly ranks the compounds in order of lowest boiling point to highest boiling point based only in intermolecular forces of CH3CH2OH CH3OCH3 and CH3CH2CH3. Rank the following compounds by viscosity from most viscous to.
Viscosity is a material property which describes the resistance of a fluid to shearing flows. Two of these 19. Ethanol has the lowest vapor pressure and strongest intermolecular force due to hydrogen bonding c.
CCl 4 l b. For instance honey has a much higher viscosity than water. In the molecule of the hydrogen bonding forces tend to resist any phase transition form liquid state to gaseous phase and these hydrogen bonding forces are greater usually when the hydrogen is bound to an electronegative atom like chlorine fluorineoxygenetc.
Which of the following compounds has the lowest viscosity. Rank the following compounds from lowest viscosity to highest viscosity. Which of the following compounds has the lowest viscosity.
H2O CCl4 B. Which of the following compounds has the highest boiling point. Measured values span several orders of magnitude.
SbBr3 AsBr5 SBr2 BeBr2 OBr2. Which of the following compounds has the lowest viscosity. Which of the following would you expect to have the lowest boiling point.
This then lowers the viscosity of the compound. SiS2 BH3 SbH3 RbBr CH3OCH2CH3. Which of the following has the lowest viscosity at a given temperature of all.
B Butane C4H10 is a gas at STP while pentane C5H12 is a liquid. Rank the following compounds by BP from lowest to highest. Add answer 10 pts.
Rank the following compounds by vapor pressure from lowest to highest. B CH₃NH₂ CH₃CH₃ CH₃SH. Water experiences hydrogen bonding so it has a higher surface tension than acetone which only has dipole-dipole interactions.
Heptane has lower vapor pressure than acetone due to London dispersion forces. Of all fluids gases have the lowest viscosities and thick liquids. Put the following compounds in order of decreasing viscosity 1 is the highest and 4 is the lowest.
Which of the compounds below is an example of a network solid. Rank the compounds in Problem 1263 in order of decreasing viscosity at a gi 0403 Of the following liquids at 20circ mathrmC which has the smallest. This problem has been solved.
Transcribed image text. Which one of the following correctly ranks the compounds in order of lowest enthalpy of vaporization to highest enthalpy of vaporization based only in intermolecular forces.

Solved Rank The Following Compounds In Order Of Increasing Chegg Com
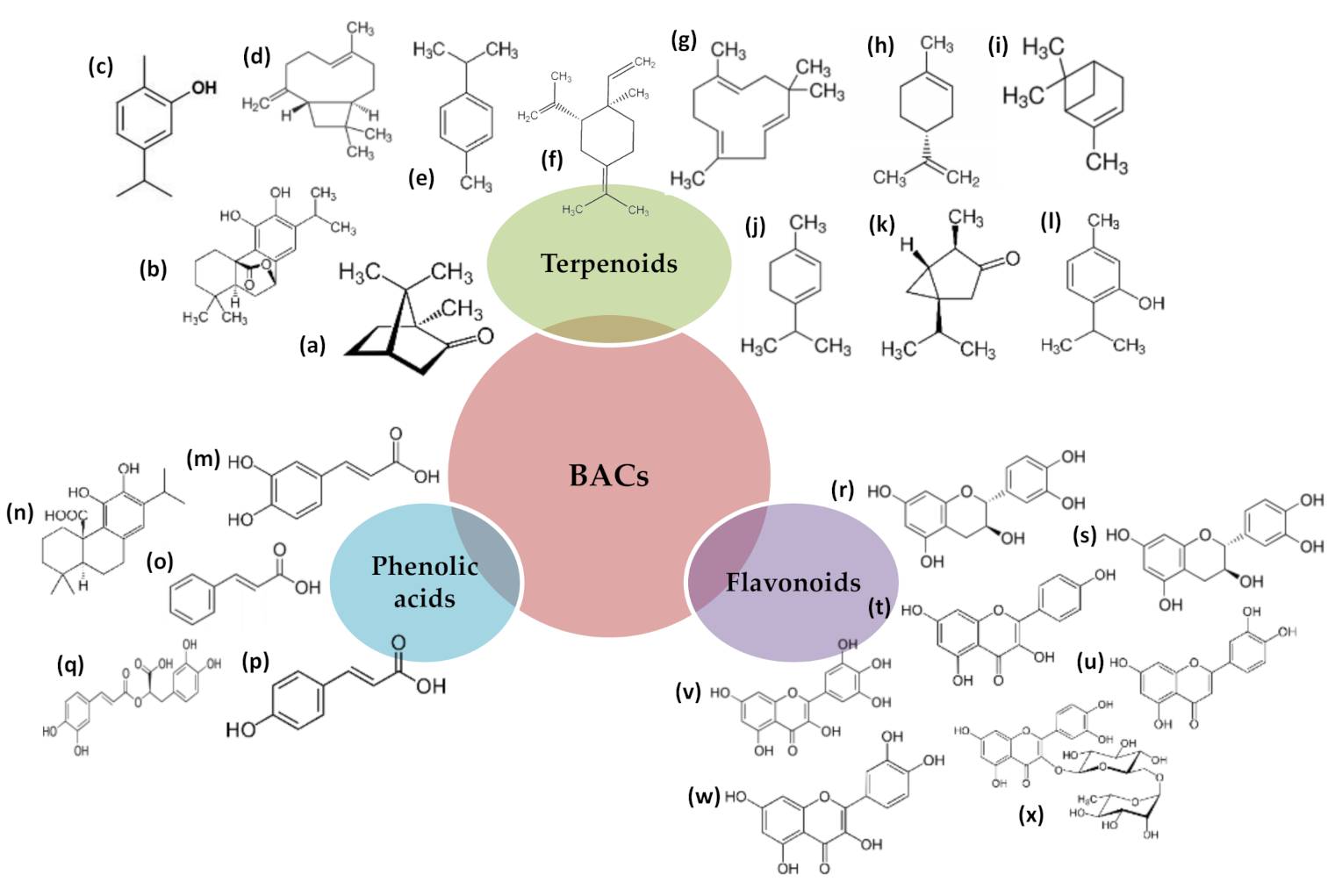
Antioxidants Free Full Text Plant Extracts Obtained With Green Solvents As Natural Antioxidants In Fresh Meat Products Html
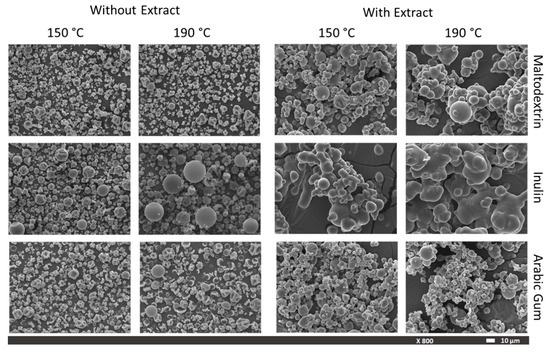
Foods Free Full Text Microencapsulation Of Pineapple Peel Extract By Spray Drying Using Maltodextrin Inulin And Arabic Gum As Wall Matrices Html
No comments for "Which of the Following Compounds Has the Lowest Viscosity"
Post a Comment